News
UC San Diego Team Uncovers Mystery of Water's Hidden Dual Phases
Published February 26, 2025
By Kimberly Mann Bruch
For decades, water’s behavior in extreme conditions has puzzled scientists. A study using U.S. National Science Foundation (NSF) ACCESS allocations on Expanse at the San Diego Supercomputer Center, part of the UC San Diego School of Computing, Information and Data Sciences (SCIDS), has produced compelling evidence that at supercooled temperatures and high pressures, water can exist in two distinct liquid states, each with its own unique structure and density.
A team led by Francesco Paesani, Kurt Shuler Faculty Scholar and professor in the Department of Chemistry and Biochemistry at UC San Diego, conducted microsecond-long simulations on Expanse over several years to explore water’s behavior under extreme conditions.
“This fascinating possibility was first proposed in 1992 by my co-author Francesco Sciortino and his colleagues—challenging conventional understanding of water’s phase behavior,” Paesani said. “Back then, researchers used a computational model and theorized that water might segregate into two liquid phases under certain conditions, but technological limitations left the idea largely speculative.”
Fast-forward to today, Paesani credits the advances in computational modeling on supercomputers like those available via the NSF ACCESS program in allowing for more precise simulation. “We now use neural networks and data-driven, many-body potentials derived from first principles to achieve an unprecedented level of accuracy in molecular simulations,” he said.
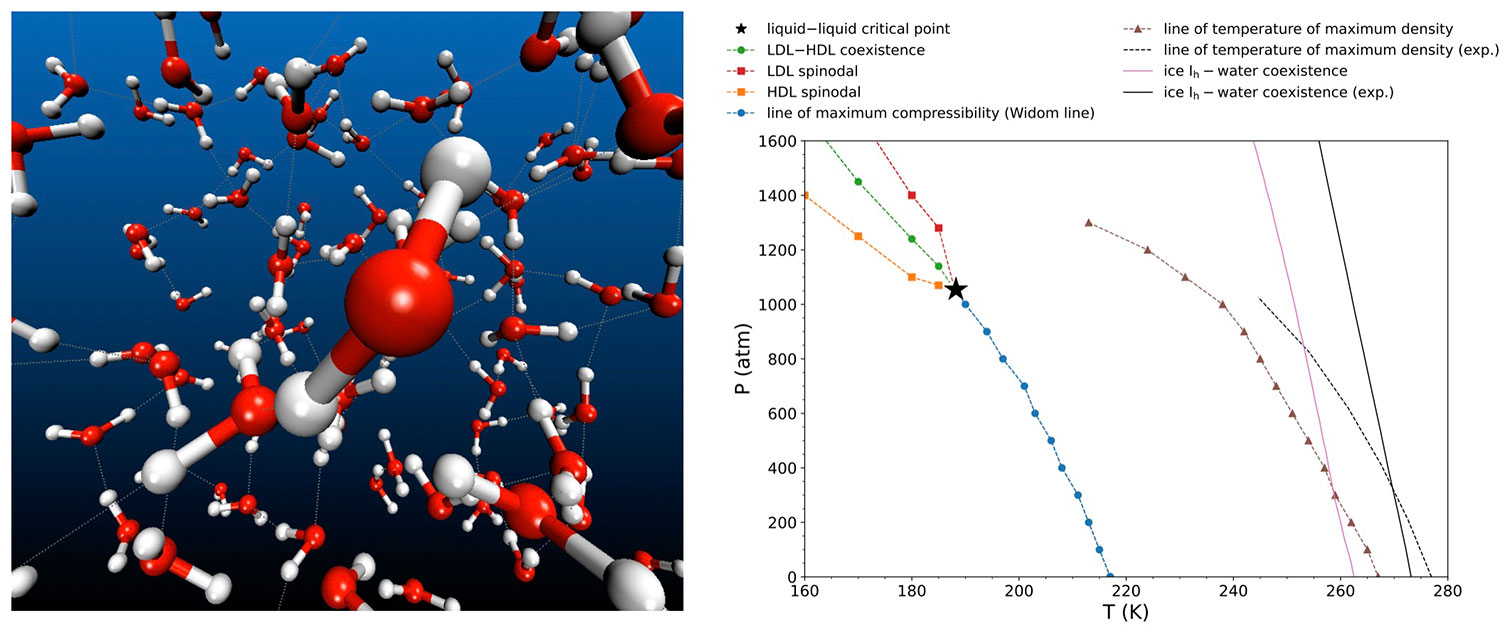
Left: Snapshot of a molecular dynamics simulation of supercooled water. Right: Phase diagram of supercooled water predicted by molecular dynamics simulations with the DNN@MB-pol potential. The predicted liquid-liquid critical point is indicated as a star at the end of the Widom line (blue), corresponding to the locus of maximum fluctuations along isobars. Also shown are the coexistence line (green) between low-density (LDL) and high-density (HDL) liquids, the LDL (red) and HDL (orange) spinodals, the calculated (brown) and experimental (dashed black) lines of maximum mass density, and the calculated (pink) and experimental (black) ice-water coexistence lines. Credit: Francesco Paesani
The team’s findings pinpoint the liquid-liquid critical point—where the two liquid phases coexist—at approximately -73°C (200 K) and 1,250 atmospheres of pressure. Paesani said that these conditions are within a range that could be tested experimentally—particularly in tiny water nanodroplets.
“Our discovery not only deepens an understanding of water’s unusual properties but also sets the stage for groundbreaking experiments,” Paesani said. “If our simulations on SDSC’s Expanse are validated in the lab, this research could have far-reaching implications for fields ranging from materials science to biology, where water plays a fundamental role.”
The study has been published in Nature Physics.
Computational support was provided by ACCESS (allocation no. CHE230052).

